Recently, the team led by He Teng, associate researcher of the Compound Hydride Materials Chemistry Research Group, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and researcher Chen Ping, cooperated with Xiamen University professor Wu An'an and Tom Autrey of the Northwest Pacific National Laboratory in the United States. New progress has been made in this respect, and related research results are published on the back cover of "German Applied Chemistry" (Angew. Chem. Int. Ed.).
Hydrogen has always been regarded as an ideal carrier for energy storage and transportation due to its high energy density and no pollution. However, the lack of a safe and efficient hydrogen storage medium is considered to be the bottleneck of hydrogen energy application technology. At present, the world's major automobile companies are using high-pressure gas tanks as a hydrogen storage system for commercial fuel cell vehicles. However, the pressure of this system is as high as 350-700 bar, and safety has been paid attention to. In addition, the tank material of the high-pressure gas tank is high-strength carbon fiber, which is expensive to manufacture, which is also one of the reasons for limiting the rapid promotion of fuel cell vehicles. Storing hydrogen in condensed matter has been a hotspot in the research of hydrogen storage materials in the past two decades, and various novel materials have been developed one after another. However, these materials have their own shortcomings. Therefore, the development of safe, efficient, and inexpensive hydrogen storage and transportation carriers will provide effective solutions for the promotion of fuel cell vehicles and the large-scale application of hydrogen energy.
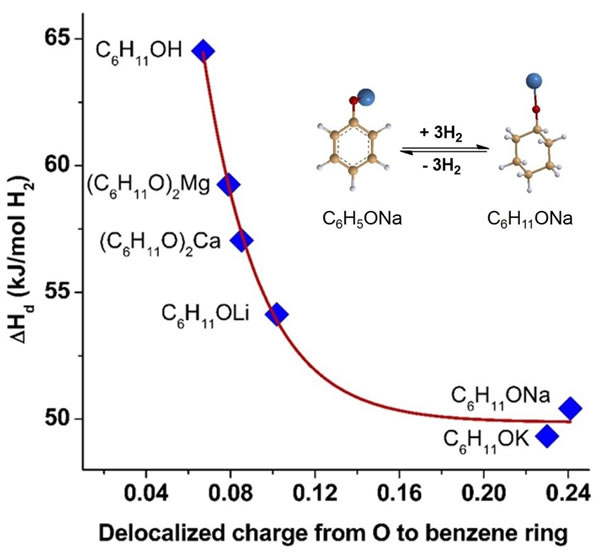
Recently, the team proposed a brand new strategy: using the difference in electronegativity of metals to modify the electronic properties of organic hydrogen storage materials, a new class of organic-inorganic hybrid hydrogen storage systems-metal organic compounds were synthesized. Theoretical calculations show that increasing the electron density in the organic carbon ring can significantly reduce the dehydrogenation enthalpy change of organic matter, and the higher the electron density, the lower the dehydrogenation enthalpy change. Researchers use alkali metals or alkaline earths with strong electron donating properties Metal-modified organic hydrogen storage materials found that the electron density in their rings increased significantly, which effectively reduced the dehydrogenation enthalpy change of organic materials; at the same time, theoretical calculations also showed that as the electron donation properties of metals increase, the dehydrogenation enthalpy of materials The lower the change, that is, by selecting different metals, the dehydrogenation enthalpy change of the material can be controllably adjusted, thereby thermodynamically controlling the dehydrogenation temperature of the material. This work takes sodium-modified phenol-cyclohexanol as an example, and it is found that the dehydrogenation enthalpy change can be reduced from 64.5kJ / mol-H2 to 50.4kJ / mol-H2; in addition, as the electron donating ability of metals increases, cyclohexane The length of the CH bond bond at the α position of sodium alkoxide increases, and the two have a linear relationship, which shows that the material has been activated after organic-inorganic hybridization, and the CH bond at the α position is preferentially broken during the dehydrogenation process.
The experimental results show that the sodium phenolate-cyclohexanol sodium system can complete the reversible hydrogen storage cycle at 150 ℃ under a commercial catalyst. After dissolving the material in water for the hydrogen storage cycle reaction, the material's hydrodehydrogenation temperature can be further reduced to below 100 ° C. Compared with the common liquid organic hydrogen storage materials, this kind of metal organic compounds can store and transport hydrogen at normal temperature and pressure, avoiding the dangers caused by high-pressure gas tanks. In addition, due to the many types and changes of organic substrates, hybridization with inorganic metals can lead to a wider variety of candidate materials for further screening. Therefore, this research has opened up new ideas for the future development of low-temperature reversible hydrogen storage materials.
This work was supported by the National Natural Science Foundation Commission Project, the Ministry of Science and Technology International Cooperation Special Project, and the Dalian Chemical Institute's independent deployment fund.
Small Motor,Tubular Motor For Blinds,Tubular Motors For Roller Blinds,Tubular Motor For Automatic Awning
Zhejiang Huzhou SCVE Machine & Motor Co., Ltd. , https://www.scve-motor.com